strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,805
Yes, the sulphate and chloride will continue to increase at the same rate, however you can't have negative alkalinity. With no bicarbonate left for the acid to react with, you should see a sharp drop in pH pretty quickly but there may also be some unwanted affects, especially if the water is in contact with metals. Although I'm not entirely sure about that, maybe an actual chemist could help there!Confusing myself over water treatment again.
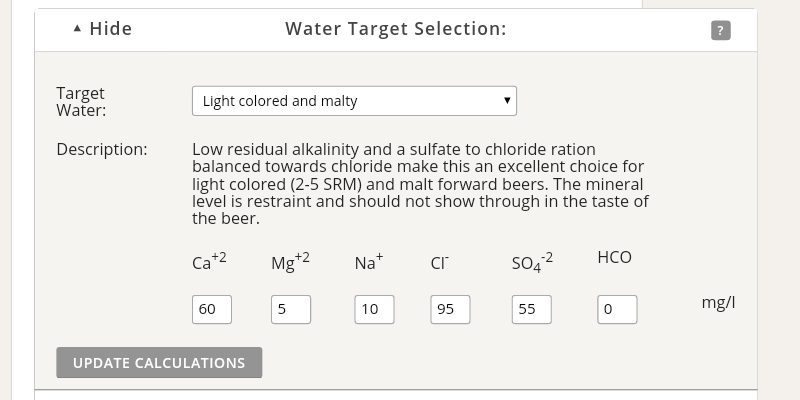
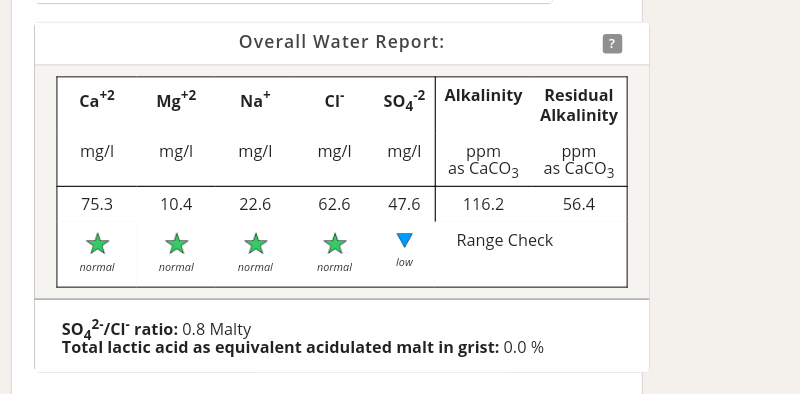
Does anyone know - if I add enough AMS to reduce alkalinity below zero, does it continue to increase the sulphate & chloride in the same way once i'm into the negatives numbers?
Is it even possible, in practise, to have a negative alkalinity?
I went down to -5 alkalinity yesterday and used 2% acidulated malt, but the mash pH was still too high
So you reduced the alkalinity to 0 with AMS, plus added 2% acid malt and the mash pH was still high? What was the pH, and the grain bill?