Hi guys, I have been doing a couple of brews recently but have been wimping out and using bottled water for them:whistle:. I would like to start understanding how to alter my own tap water better and been looking at Palmerââ¬â¢s how to brew guide.
Iââ¬â¢m not going to give out my actual postcode but if anyone wants to check a local water report lets just use ââ¬ÅME7 3PDââ¬Â as itââ¬â¢s close enough. The problem I am finding is that Southern Water does not seems to give the magnesium values for the water making it hard (or impossible???) to use Palmer's PH adjustment table.
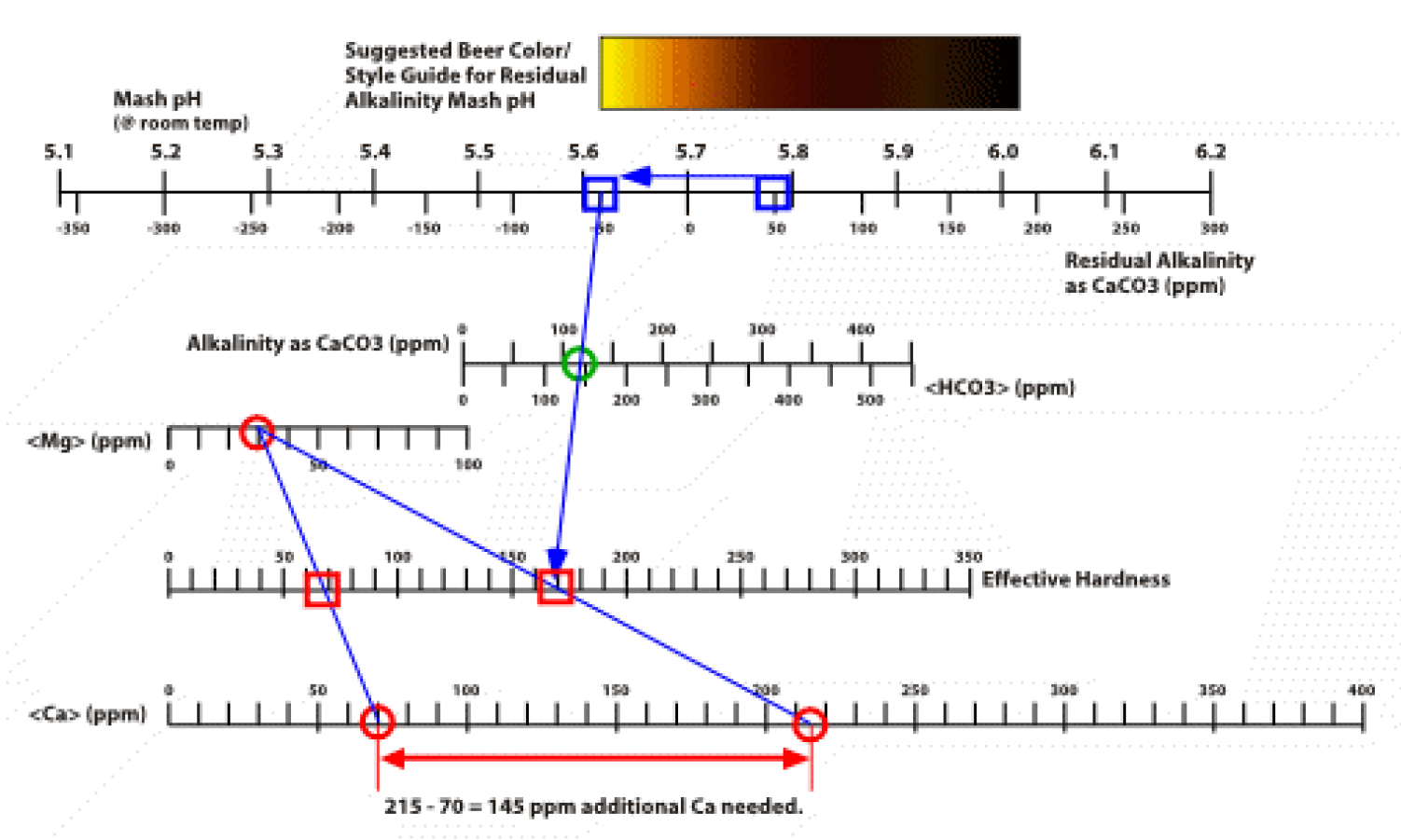
To combat this issue I would uses the value given for CaCO3 (assuming this to be total hardness as both Ca and Mg) to back calculate the Mg value, my understanding from Palmer is:
Total hardness as CaCO3 is Ca as CaCO3 plus Mg as CaCO3.
The values given by Sothern are:
CaCO3 = 243mg/l (or PPM) this is not specified as total or anything.
Ca = 97.2mg/l
Palmer states Ca hardness as CaCO3 = Ca (PPM) /20*50
Using Ca =97.2mg/l or PPM
CaCO3 from calcium=97.2/20*50 =243mg/l
From the total harness equation I assume CaCO3 total = Ca/20*50 + mg/10*50
With my CaCO3 value from Ca alone equaling 243 this would mean zero mgââ¬Â¦
So have I messed up or has southern water (assuming i have missed something here)ââ¬Â¦?
P.S. Sorry if this is really hard to followââ¬Â¦ Also, sorry if my chemistry is completely flawed
Iââ¬â¢m not going to give out my actual postcode but if anyone wants to check a local water report lets just use ââ¬ÅME7 3PDââ¬Â as itââ¬â¢s close enough. The problem I am finding is that Southern Water does not seems to give the magnesium values for the water making it hard (or impossible???) to use Palmer's PH adjustment table.

To combat this issue I would uses the value given for CaCO3 (assuming this to be total hardness as both Ca and Mg) to back calculate the Mg value, my understanding from Palmer is:
Total hardness as CaCO3 is Ca as CaCO3 plus Mg as CaCO3.
The values given by Sothern are:
CaCO3 = 243mg/l (or PPM) this is not specified as total or anything.
Ca = 97.2mg/l
Palmer states Ca hardness as CaCO3 = Ca (PPM) /20*50
Using Ca =97.2mg/l or PPM
CaCO3 from calcium=97.2/20*50 =243mg/l
From the total harness equation I assume CaCO3 total = Ca/20*50 + mg/10*50
With my CaCO3 value from Ca alone equaling 243 this would mean zero mgââ¬Â¦
So have I messed up or has southern water (assuming i have missed something here)ââ¬Â¦?
P.S. Sorry if this is really hard to followââ¬Â¦ Also, sorry if my chemistry is completely flawed


















![BREWING THERMOMETER STICKERS ACCURATELY MONITOR FERMENTING BEER & WINE LIQUID TEMPERATURES 5PCS HOME BREW SPIRITS WINE LCD ADHESIVE [US]](https://m.media-amazon.com/images/I/311DDjo2X3L._SL500_.jpg)























