Cheers for help understand it lot better now much appreciatedNo, the stars don't mean you're close to your target, they only mean the values are within recommended general brewing range. When the numbers in the "difference" row go green, then you're close enough.
For a NEIPA the closest is probably light and malty, but personally I'd increase the calcium from 60 to 100 ppm.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
RO system
- Thread starter liamf89
- Start date

Help Support The Homebrew Forum:
This site may earn a commission from merchant affiliate
links, including eBay, Amazon, and others.
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,805
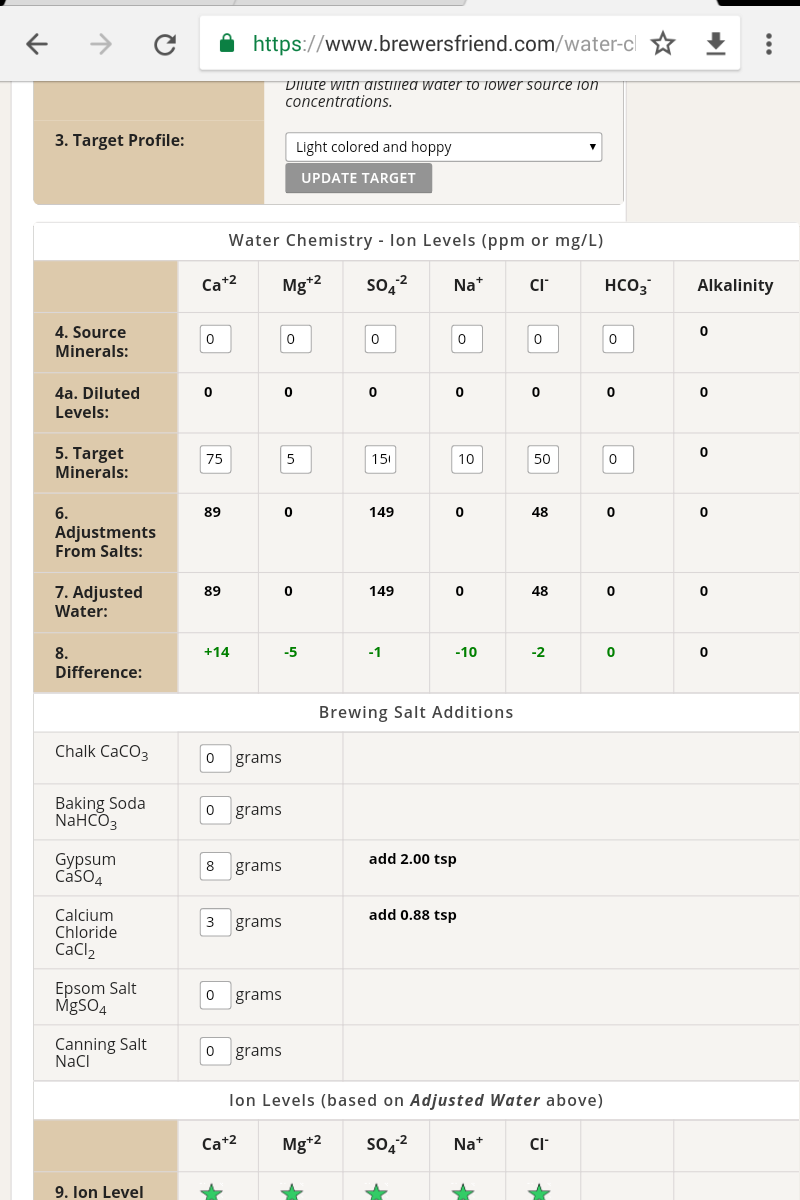
Oops, try this one. This is for a water volume of 30L:Hi Steve. I made the mistake of thinking the software would calculate the additions for you but now i have a better grasp on it. Any chance you could upload that screenshot again as it too low res to read.
I bought this one, very pleased with ithttps://rover.ebay.com/rover/0/0/0?mpre=https://www.ebay.co.uk/ulk/itm/122094639916 how does this one look okay do you think
https://www.ebay.co.uk/itm/Finerfil...e=STRK:MEBIDX:IT&_trksid=p2057872.m2749.l2649
Well was having a wee look for water for neipa i remember you said make so 200 does this seem about right is for thirty litresOops, try this one. This is for a water volume of 30L:

Attachments
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,805
I changed the sulphate to 75 and chloride to 150 put can't seem to get the calcium at the right number is always to high am only trying to add calcium chloride and gypsum to get right figures added 3gram gypsum 9.5 calcium chloride will I need add another salt to get this too right figure and lower calcium thanks@liamf89 for a NEIPA you want to reverse the sulphate:chloride ratio, something like 75ppm sulphate and 150ppm chloride would be a decent starting point.

£14.66 (£147.75 / kg)
Spicy World Tartaric Acid Pure 3.5oz - Top Grade, USP Certified, Food Grade, Vegan - Perfect for Wine Making, Mead
Amazon US

£63.25
HJBFZZBD Sanitary clamp 1.5" (38mm) FlangeOD50.5mm Sanitary Tri-Clamp 90-Degree Pipe With Middle Nipple And Electronic Thermometer, Stainless Steel 304 Home brewing(Without Thermometer)
DAN CHENG XIAN PENG SHI DA SHANG MAO DIAN

£11.95
£14.99
WATER COMPREHENSIVE GUIDE (Brewing Elements): A Comprehensive Guide for Brewers
Amazon.co.uk

£63.25
Sanitary clamp 1.5" (38mm) OD50.5mm Sanitary Tri-Clamp 90-Degree Pipe With Nipple And Electronic Thermometer, Stainless Steel 304 Home brewing(Without Thermometer)
DAN CHENG XIAN PENG SHI DA SHANG MAO DIAN

£14.25
£18.99
How to Brew: Everything You Need to Know to Brew Great Beer Every Time
Amazon.co.uk

£6.89 (£344.50 / kg)
£7.51 (£375.50 / kg)
2x Mangrove Jack’s Craft Series Mead Yeast M05 (10g)
do-it-at-home

£437.77
HMCOCOOFM 4L 4" OD119mm * 1.5" OD50.5mm Copper Onion Head For Homebrewing,Thickness 1.5mm (With Thermometer)
weifangguanhuawangluokejiyouxiangongsi

£32.95
£34.95
DIAH DO IT AT HOME Beer & Wine Making Starter Kit - Basic Equipment - All You Need in One Box Homemade Beer & Wine Home Brewing
do-it-at-home

£14.76
Digital Temperature Watch Heating Thermometer Home Brewing Tools for Wine Bottle
B&D DIRECT STORE

£18.95
£20.89
BALLIIHOO 30 Litre Homebrew and Winemaking Fermentation Bucket with Lever Tap & LCD Temperature Strip
BalliihooHomebrew

£73.98
Sanitary clamp 2" (51mm) OD64mm Sanitary Tri-Clamp 90-Degree Pipe With Nipple And Electronic Thermometer, Stainless Steel 304 Home brewing(Without Thermometer)
DAN CHENG XIAN PENG SHI DA SHANG MAO DIAN

£15.96
£16.99
The Brew Your Own Big Book of Clone Recipes: Featuring 300 Homebrew Recipes from Your Favorite Breweries
Amazon.co.uk

£10.49 (£349.67 / kg)
£11.96 (£398.67 / kg)
Mangrove Jack 3X ’s Craft Series Mead Yeast M05 (10g)
Almost Off Grid

£13.58
Banziaju Wine Making Supplies, 18" Auto Siphon Hose For Water Homebrew Siphon Pump With Tubing And Clamp Clear Wine Siphon For Beer Wine Making Kit
Jian Shi Xian Chao Mei Shang Mao You Xian Gong Si
I just looked back seen you recommend have calcium at 100 so that sorts my problem there thanks@liamf89 for a NEIPA you want to reverse the sulphate:chloride ratio, something like 75ppm sulphate and 150ppm chloride would be a decent starting point.
- Joined
- Mar 22, 2019
- Messages
- 1,127
- Reaction score
- 572
For RO water you might use these measurements for burton on trent profile? Right? or does it need some ph balancing too


strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,805
Well this was a blast from the past, it was nice seeing some posts from my old mate Johnnyboy1965. I miss his posts where his anger was matched only by his ignorance.
Anyway @Justin Dean, personally I would never use that profile, the calcium, sulphate, and bicarbonate are much too high for me.
Anyway @Justin Dean, personally I would never use that profile, the calcium, sulphate, and bicarbonate are much too high for me.
- Joined
- Mar 22, 2019
- Messages
- 1,127
- Reaction score
- 572
Yes mate, I agree mate, but the calculation is right?
strange-steve
Quantum Brewer
- Joined
- Apr 8, 2014
- Messages
- 6,027
- Reaction score
- 5,805
Are you asking if those additions to DI water will give you that profile?Yes mate, I agree mate, but the calculation is right?
Well sodium bicarbonate is 27.4% by weight sodium and 72.6% bicarbonate, so 10g will add 2.74g sodium and 7.26g bicarbonate.
Gypsum is 23.3% calcium by weight and 55.8% sulphate, so an addition of 25g will add 5.58g of calcium and 13.95g sulphate.
Calcium chloride (dihydrate) is 27.2% calcium by weight and 48.2% chloride, so an addition of 5g will add 1.36g of calcium and 2.41g chloride.
Epsom salt is 9.9% magnesium by weight and 40% sulphate so an addition of 10g will add 0.99g of magnesium and 4g of sulphate.
So your total additions are:
2740mg sodium
7360mg bicarbonate
6940mg calcium
17950mg sulphate
2410mg chloride
990mg magnesium
Divide these by your volume in litres to get ppm. If you used a volume of about 25L in your calculation then, yes the numbers look about right.
Last edited:
- Joined
- Mar 22, 2019
- Messages
- 1,127
- Reaction score
- 572
Thank you mate, I was just fiddling with the numbers to get them all green because I figure that is how the calculator works, Your explanation gives me confidence that using the simple calculator in this way works. Good enough for me. The only other question is ph form ro so will look at the advanced calculator now. Learning curve step 2.
The picture above is pretty drastic from RO to Burton on Trent.
The picture above is pretty drastic from RO to Burton on Trent.
Similar threads
- Replies
- 16
- Views
- 826
- Replies
- 20
- Views
- 3K





















![BREWING THERMOMETER STICKERS ACCURATELY MONITOR FERMENTING BEER & WINE LIQUID TEMPERATURES 5PCS HOME BREW SPIRITS WINE LCD ADHESIVE [US]](https://m.media-amazon.com/images/I/311DDjo2X3L._SL500_.jpg)





