Is there anything to watch out for using lactic acid to reduce alkalinity?
I'm finally getting round to planning my first brew with the local St Albans tap water (Ca 145, Na 30, HCO3 333, SO4 42, Cl 55, NO3 31). I thought I'd aim for a 'London' profile in order to brew a Best Bitter style ale using WY1469 - comments are welcome as I'm still very new at this.
I need to get the alkalinity down, but it seems that doing this with just AMS might push the sulphate and chloride a bit high. So I think I need to use a combination of acids. I've heard phosphoric works well but I couldn't get any... however I've managed to get my hands on some un-perfumed 80% lactic.

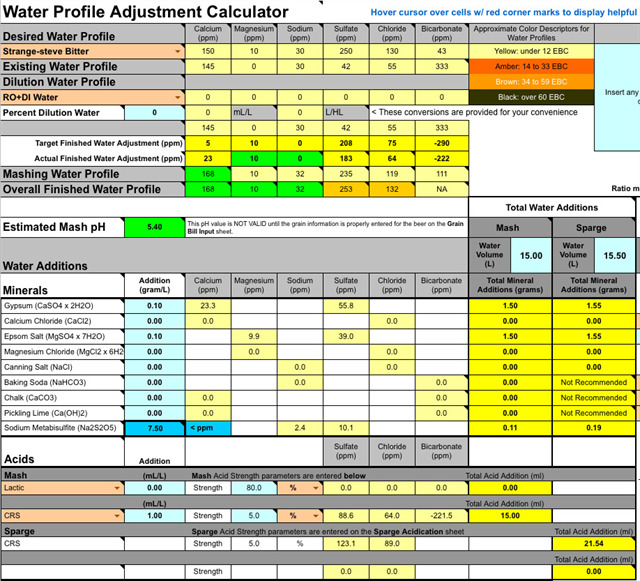
Bru'n water seems to suggest that adding roughly equal volumes of AMS and lactic will do the trick for the profile (see below); but has anyone else tried using it and is it likely to add any weird flavours or other effects? Thanks for any tips...

I'm finally getting round to planning my first brew with the local St Albans tap water (Ca 145, Na 30, HCO3 333, SO4 42, Cl 55, NO3 31). I thought I'd aim for a 'London' profile in order to brew a Best Bitter style ale using WY1469 - comments are welcome as I'm still very new at this.
I need to get the alkalinity down, but it seems that doing this with just AMS might push the sulphate and chloride a bit high. So I think I need to use a combination of acids. I've heard phosphoric works well but I couldn't get any... however I've managed to get my hands on some un-perfumed 80% lactic.

Bru'n water seems to suggest that adding roughly equal volumes of AMS and lactic will do the trick for the profile (see below); but has anyone else tried using it and is it likely to add any weird flavours or other effects? Thanks for any tips...







![BREWING THERMOMETER STICKERS ACCURATELY MONITOR FERMENTING BEER & WINE LIQUID TEMPERATURES 5PCS HOME BREW SPIRITS WINE LCD ADHESIVE [US]](https://m.media-amazon.com/images/I/311DDjo2X3L._SL500_.jpg)