Dear experts!
I'm finding it hard to increase my SO4/Cl ratio without sending the Ca and Mg concentration too high.
It looks like adjusting the pH with sulphuric acid would provide a great answer, but unfortunately due to the actions of a few execrable lunatics it seems to be all but impossible to buy these days.
I'm wondering whether it's a viable and/or advisable alternative to add slightly more sodium meta-Bi (Camden). If my (limited!) understanding is correct, then the normal dose of 1/2 a tablet in 30L of water is about 7.7ppm but if I take this up to 15ppm then it contributes a useful amount of sulphate without raising chloride...
Any comments are really, really welcome - many thanks in advance.
The gory details
My water report says that I have:
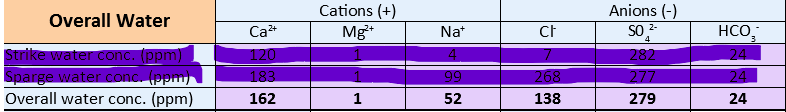
Ca 145, Mg 0, Na 30, SO4 42, Cl 55, HCO3 333
And I'm aiming for something like:
Ca 150, Mg <40, Na < 50, SO4 350, Cl 71, HCO3 155 (i.e. SO4/Cl approx 5.5)
I'm thinking of doing something like this (note also the addition of CRS and 50% dilution with RO+DI water)

I'm finding it hard to increase my SO4/Cl ratio without sending the Ca and Mg concentration too high.
It looks like adjusting the pH with sulphuric acid would provide a great answer, but unfortunately due to the actions of a few execrable lunatics it seems to be all but impossible to buy these days.
I'm wondering whether it's a viable and/or advisable alternative to add slightly more sodium meta-Bi (Camden). If my (limited!) understanding is correct, then the normal dose of 1/2 a tablet in 30L of water is about 7.7ppm but if I take this up to 15ppm then it contributes a useful amount of sulphate without raising chloride...
Any comments are really, really welcome - many thanks in advance.
The gory details
My water report says that I have:
Ca 145, Mg 0, Na 30, SO4 42, Cl 55, HCO3 333
And I'm aiming for something like:
Ca 150, Mg <40, Na < 50, SO4 350, Cl 71, HCO3 155 (i.e. SO4/Cl approx 5.5)
I'm thinking of doing something like this (note also the addition of CRS and 50% dilution with RO+DI water)



























![BREWING THERMOMETER STICKERS ACCURATELY MONITOR FERMENTING BEER & WINE LIQUID TEMPERATURES 5PCS HOME BREW SPIRITS WINE LCD ADHESIVE [US]](https://m.media-amazon.com/images/I/311DDjo2X3L._SL500_.jpg)