But White Labs themselves recommend the use of a stir plate for making yeast starters...
I find it hard to believe that they would be sucked into perpetuating a homebrew myth if there were not some evidence to support it
First off, the information on Kai's site needs to be viewed with the same skeptical eye with which many forum members are viewing my information. While some of the information is very good, some of it is also incorrect to the point of being very bad practice. For example, the way Kai demonstrates making plates is completely wrong. One never processes plates in a pressure cooker/autoclave. That is surefire way to make plates that are mold factories, especially in a home brewing lab. The correct way to pour plates is to dry sterilize the glass petri dishes in an oven at 170C for an hour. The media is placed in a covered glass container and processed in pressure cooker/autoclave at 15psi for 15 minutes and allowed to cool to between 49 and 60C before carefully lifting one side of the lid on a petri dish up only far enough to be able to pour enough agar-based media to cover the bottom of the dish before putting the cover back down. This technique works with pre-sterilized plastic plates as well. It results in significantly less condensation forming on the lids of the petri dishes. The condensation is insignificant enough that it will evaporate during proofing. Why did Kai not know that before writing his article? While Kai is clearly a very bright guy, it kind of calls into question his microbiology cred.
The White Labs video confounds me as well because it is not a practice White Labs uses in-house. White Labs does not use stir plates for in-house culturing. They use a sizeable multi-level orbital shaker in their main starter propagation room with shaker flasks. Shaker flasks resemble Erlenmeyer flasks, but they have baffles in the bottom to create turbulence and are usually squatter to improve air/liquid specific surface area. Some people use Erlenmeyer flasks with an orbital shaker, but the correct piece of lab glassware is the shaker flask. The funny thing is that Chris White stated that amateur brewers tend to be way too wrapped around the axle with respect to cell counts when I discussed the use of stir plates and yeast in general with him at NHC San Diego in 2015.
The reality is that no matter what is claimed a culture cannot exceed maximum cell density per ml because yeast cultures are self limiting. We are talking about viable in cells when we are discussing maximum cell density. If a starter reaches high krausen (i.e., a solid head forms on it) before it exhausts the carbon sources in the wort (sugar is carbon bound to water), it can be assumed to have reached maximum cell density for the volume of wort in the starter because high krausen occurs when a yeast culture reaches maximum cell density (i.e., high krausen marks the end of the exponential growth phase). Maximum cell density is based on actual yeast cell size (some yeast cells are larger than others), but a general rule of thumb is 200 million cells per ml, which means that a 1L starter that reaches high krausen can be assumed to have 200 billion cells. If we pitch the entire starter at high krausen into 23L of work, we end up with 24L of wort. If that bothers people, then they should concentrate their wort enough to allow for the dilution because pitching at high krausen results in a significant reduction in lag time, which is part of the secret sauce of SNS. Pitching the entire contents of a starter does not bother me, and I only pitch into 20L of wort most of the time, so the dilution is greater. I just adjust for it by having a slightly higher end of boil gravity than my target original gavity.
Now, getting back to our 200 billion cells in what is now 24L of wort. That number of cells yields a cell density of 200,000,000,000 / 24000 = ~8.33 million cells per milliliter. Using George Fix's rule of thumb that one should pitch 750,000 cells per ml of wort per degree Plato, 8.33 million cells per milliliter is good enough to pitch 8.33 / 0.75 = 11.11 Plato (~1.045 S.G.). However, in practice, a 1L starter pitched at high krausen will handle worts up to 16 Plato (1.065) with ease, that is, as long as we have a sound wort aeration.
With that said, we have to be more careful with high gravity wort because of two things; namely, much higher osmotic pressure and the increased difficulty of dissolving O2; therefore, affecting plasma cell membrane health if too much exponential growth is required to reach maximum cell density for a batch or wort. Higher gravity wort has a lower O2 saturation threshold for any given temperature than lower gravity wort, which means that we cannot support as much healthy cell growth. Osmotic pressure is phenomenon where water is drawn to the side of a semi-permeable membrane (the yeast cell cell plasma membrane in this case) with the highest solute content. Wort can be thought of as a solvent (water) and a solute (malt sugars) that is dissolved into the solvent. The solute on the wort side of the cell wall is much higher than it is inside of the cell, which results in water being drawn out of the cells, leading to the loss of cell membrane turgor pressure, which, in turn, results in cell shrinkage and wrinkling, and if taken to an extreme, cell implosion. There is also the additional threat posed to the culture by the higher alcohol levels found in high gravity beers. The page shown below is from page 220 in an article entitled "The Effects of Osmotic Pressure and Ethanol on Yeast Viability" in an issue of the Journal of the Institute of Brewing.
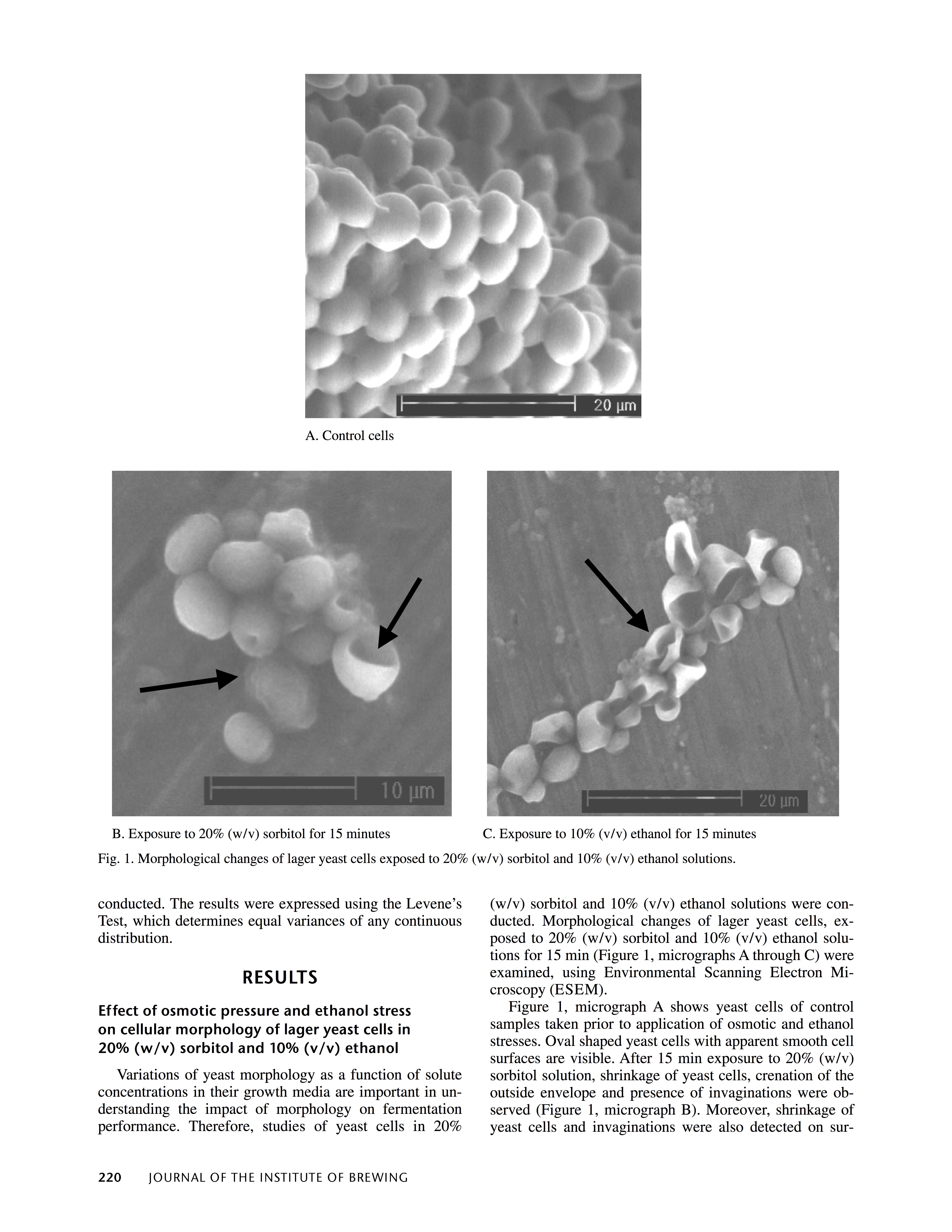
The image on the bottom left is what lager yeast cells look like when subjected to a 20% w/v (an S.G. of 1.083) sorbitol solution. As one can see, the cells have indentations from the loss of turgor pressure. The image on the right is what happens after yeast cells are exposed to 10% ethanol.
For the forum member who wants a detailed explanation of the SNS method, here is a link to the original SNS post I made on Jim's Beer Kit back in 2015:
Shaken, not Strirred - Home Brew Forum
For those who want cell counts with respect to SNS, once again, SNS is about the fact that yeast cultures do not need to be spun, they need dissolved O2 at the time of pitching. SNS is based on a physical property of foam that allows one to diffuse a significant amount O2 into wort in a low-tech way coupled with two rules of thumb; namely, the maximum cell density for a 1L starter can be assumed to be 200 billion cells and pitching at high krausen results in optimal use of the ergosterol and unsaturated fatty acids (UFA) reserves that were built by the mother cells that were alive during a starter's lag phase. By pitching at high krausen, we avoid wasting ergosterol on replacement-only replication (remember, yeast cultures are self-limiting; therefore, they only replicate to replace cells that have died after maximum cell density has been reached). That is what occurs if we allow a starter to ferment past high krausen, and that is what most people who are using stir plates are doing. They are wasting ergosterol and UFA reserves that will need to be replenished when the culture is pitched into wort, driving up dissolved O2 demand. Not only that, allowing a starter for ferment out results in morphological changes that need to be reversed during the lag phase, which, in turn, lengthens the lag phase.
In the end, people are going to believe what they want to believe. Nothing I write is going to convince anyone with a closed mind. It does not bother me. I have been swimming upstream for quite a while and I only stand my ground when I know I am right. The shear number of people who have tried SNS and either parked or gave away their stir plates is evidence enough that the method is based sound principles. I stand on my belief that yeast calculators attempt to impose precision where none exists. Rules of thumb are good starting points, but only experience with a culture in one’s brewery teaches one what is needed. Like most breweries, I never pitch high gravity wort with a starter. I always use slurry harvested from a healthy standard gravity batch. A yeast culture almost always performs better on a second or third pitch than it does on the first pitch. That is what is known as a brewery best practice.
 , but I do like the simplicity of a "Shake n Bake" approach. I say if they are both achieving the same quality of beer, that the brewer enjoys, then let em have at it. I am glad to have come across this though and feel both methods should be side by side in books for the homebrewer to choose
, but I do like the simplicity of a "Shake n Bake" approach. I say if they are both achieving the same quality of beer, that the brewer enjoys, then let em have at it. I am glad to have come across this though and feel both methods should be side by side in books for the homebrewer to choose , but I do like the simplicity of a "Shake n Bake" approach. I say if they are both achieving the same quality of beer, that the brewer enjoys, then let em have at it. I am glad to have come across this though and feel both methods should be side by side in books for the homebrewer to choose
, but I do like the simplicity of a "Shake n Bake" approach. I say if they are both achieving the same quality of beer, that the brewer enjoys, then let em have at it. I am glad to have come across this though and feel both methods should be side by side in books for the homebrewer to choose







































